
If they're all divisible by a number, say 2, go ahead and do that to reduce the coefficients to their lowest-possible whole-number (integer) values. When you're done balancing, take a look at your coefficients. This last step could spoil one of the other elements I've just balanced, so be aware of that - you might have to start over, but not this time: OK, so that's 6 carbons and 6 hydrogens on each side. Now the hydrogens: I've got six on the left, so I'll have to multiply H 2O by 3: I'll start with the carbons (a random choice, but there's probably some "intuiting" going on in my brain because I've done this many times): How many atoms of aluminum are on each side of the following equation: 4Al + 3O 2 -> 2Al 2 O 3. At the end of chemical reactions, what is the total mass of the reactants compared to the total mass of the products Q. So far as I can recall, I've never balanced this equation before now:īoth sides have a moeity with an odd number of oxygens, so I'm not overly concerned about multiplying everything by two right now, though I might have to later, who knows?. True or False: Chemical equations show how atoms are rearranged in a chemical reaction. What follows is just my train of thought as I write this. Now, however, we see that there are 4 aluminums on the product side, so again we need to adjust the reactant aluminum! We can then change the coefficient of 2 in front of the aluminum to a 4.įinally we have a balanced equation: 4Al + 3O2 -> 2Al2O3. Traditional matrix-inversion methodologies for balancing chemical-reaction equations can be used to balance only a restricted class of equations (i.e. We can then adjust each side to have 6 oxygens by putting a coefficient of 3 in front of the diatomic reactant oxygen and a 2 in front of the compound on the product side. There are 2 on the reactant side and 3 on the product side – since neither one is a multiple of the other, the best thing to do is find the least common multiple of both of them. How will we balance it?įirst, let’s multiply the aluminum on the reactant side by 2 to match the amount of aluminum on the products side. Add coefficients (the numbers in front of the formulas) so the number of atoms of each. Write down how many atoms of each element there are on each side of the reaction arrow. Follow four easy steps to balance a chemical equation: Write the unbalanced equation to show the reactants and products. We can see that this is not balanced – there is 1 aluminum on the reactant side and 2 on the product side, and there are 2 oxygens on the reactant side and 3 on the product side. Easy Steps for Balancing Chemical Equations. Balancing Equations: Practice Problems 1. Let’s try an example! We will take the given equation: Al + O2 -> Al2O3. Remember, ferric chloride and sodium chloride are soluble in water. Balancing equations calculator performs as to balance the given equation, it calculates the coefficients also. It is an online tool which works digitally and provide quick results. Balancing chemical equations calculator works in sensible manners as there is artificial intelligence is doing work. The balanced equation between the two would look like this: Fecl3 + 3NaOH Fe (OH)3 + 3NaCl. Introduction of Chemical Equation Balancer. Essentially you are multiplying the amount of atoms or compounds on one side to match the amount on the other side. Before balancing the equation, let’s be clear about each chemical formula: Ferric chloride- FeCl3. To balance an equation: we add a coefficient to the front of each element or compound that requires it.

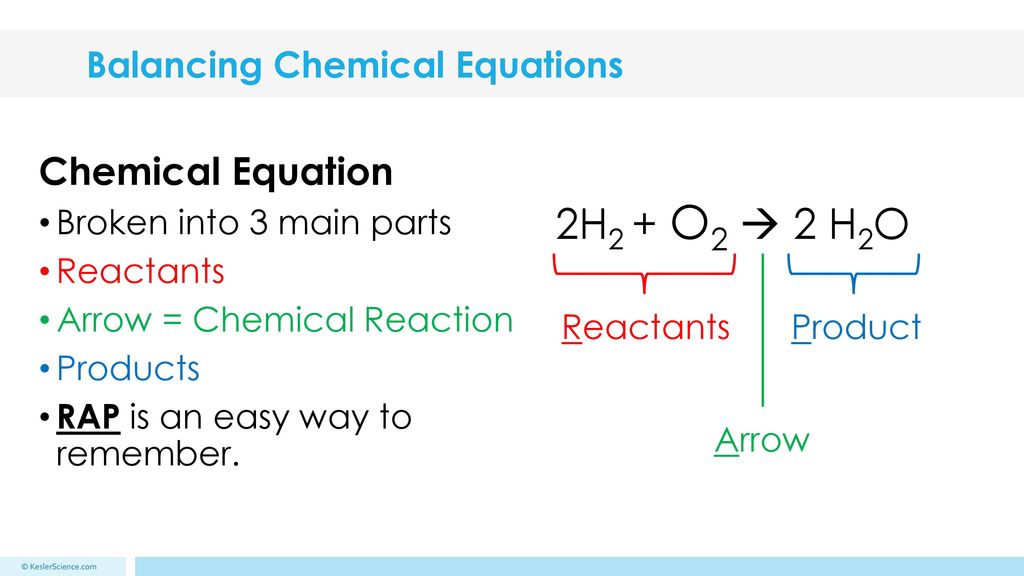
Here is an example of a chemical equation: Al + O2 -> Al2O3.Ĭhemical equations must be balanced since anything that is consumed by the reaction must form product – we can’t have atoms disappearing or appearing out of nowhere! A chemical reaction can be expressed as an equation.Ī chemical equation shows the chemicals that react (the reactants) followed by an arrow followed by the chemicals that are produced by the reaction (the products).


 0 kommentar(er)
0 kommentar(er)
